The Australian COVID-19 Vaccine Claims Scheme
Misalignment between the evidence base and Scheme design
Professor Gemma Carey
Rachel O’Reilly
Dr Rado Faletič
Dr Nicholas Bromfield
Professor Dilan Thampapillai
Foreword by Dr Monique Ryan, Federal Member for Kooyong
All medicines have side-effects – COVID vaccines were no exception. With vaccines developed with great haste, at the height of a global pandemic, it was always to be expected that the new agents produced against COVID would cause adverse effects of some type. It was for this reason – and because of the urgent need to secure an adequate supply of vaccines in a competitive market – that the Morrison government agreed to indemnify vaccine manufacturers against injuries arising from their agents. It then announced a plan to establish a compensation scheme for Australians who suffered an injury referable to those vaccines.
Issues with that scheme are documented in the report released this week by COVERSE / University of NSW. The report describes the vaccine-related serious adverse effects reported in Australia, and the slow and inadequate response of the vaccine claims scheme. The fact that the scheme’s end is imminent means that any late side-effects of the vaccine may go undetected. The overlap between many vaccine side-effects and the symptoms of long COVID – identified in Australia and in many other jurisdictions – will not be further studied through this scheme.
The vast majority of Australians experienced vaccination against COVID without significant ill-effects, and their lives were improved by that process. Our government owes a debt to those few who suffered injury from those medications. Surveillance and compensation schemes are important for ensuring trust in the system and accountability of institutions. Rather than closing this one down, it should be revised and re-funded, as a measure of faith to all Australians who have participated in this unprecedented vaccination program, and of ongoing support to those who continue to experience adverse effects from COVID vaccination.
Synopsis
In this report, we present findings from a systematic review of the literature on adverse events to COVID-19 vaccinations and compare this evidence base with the categorisation of approved reactions for compensation in Australia. The research looked at the extent of alignment between the COVID-19 Vaccine Claims Scheme and the available evidence on serious COVID-19 vaccine adverse events. Over 700 serious adverse events documented in the medical peer-reviewed case study literature were reviewed. Our review demonstrates a gross misalignment between the very limited approved range of serious adverse events included in the Australian compensation scheme, and the medical evidence, which shows a very wide range of serious adverse events. The vast majority of medically documented serious adverse events uncovered in the review are excluded for compensation by the Australian COVID-19 Vaccine Claims scheme. Consequently, it is reasonable that the majority of citizens experiencing serious adverse events from COVID-19 vaccines are excluded from compensation.
The report shows that the Australian Government is not providing a meaningful COVID-19 vaccine compensation scheme, and is not currently accountable for the empirical range of serious adverse events including disabilities caused by COVID-19 vaccines. We call for an immediate review of the COVID-19 Vaccine Claims Scheme to ensure it encompasses protection from injury by serious adverse events in a way that is comprehensive, equity-driven, genuinely accessible, and evidence-led.
Introduction
More than 65 million doses of COVID-19 vaccinations have been given in Australia (Ward, 2023). In 2021, alongside the rollout of the COVID-19 national vaccination program, the Australian Commonwealth Government established a compensation scheme for people who experienced significant adverse reactions to COVID-19 vaccines (Department of Health and Aged Care, 2023). At the Scheme’s launch, the then Minister for Health Greg Hunt (2021) stated that the Scheme would be “administered by Services Australia and will provide Australians with a single front door to a simple and quick administrative process for compensation”.
Citizens who have applied to the scheme have found it to be anything but simple and quick, with many denied access, or otherwise experiencing considerable administrative burdens and psychological stress (Ward, 2023). At present, only 5% of claims have been granted (Senate Community Affairs Committee, 2024).
This report provides a review of the medical evidence on the scope of serious adverse events following COVID-19 vaccines and the degree of alignment of this scope with the Australian COVID-19 Vaccine Claims Scheme. It is based on research undertaken by the Centre for Social Impact at the University of New South Wales (full research paper can be found at https://ssrn.com/abstract=4930889).
The research finds a significant misalignment between the peer-reviewed medical evidence on serious adverse events, and the design of the compensation scheme. We call for a full and urgent review of the compensation scheme to realign it with the evidence on adverse events following COVID-19 vaccines, which demonstrate a much wider array of serious adverse events than what is covered by the compensation scheme.
Vaccine compensation schemes
Vaccine compensation schemes first emerged in the 1950s with the rollout of the smallpox vaccine, and proliferated in the 1990s in the context of a broader US-led deregulation of the pharmaceutical industry, and as an alternative to tort law processes (Halabi et al., 2023). In the US context, rising lawsuits and consequential waning capital and social investments in vaccines informed the National Childhood Vaccine Injury Act of 1986 and the Vaccine Adverse Event Reporting System (HRSA, 2024). Such schemes uniformly recognise that, like most drugs, vaccinations can cause harm for some individuals (Halabi et al., 2023). Australia has been somewhat anomalous globally, only creating its first vaccine compensation scheme during the COVID-19 pandemic (Wood et al., 2020).
As Halabi et al (2023: 62) suggest, “the relationship between immunization’s critical role for public health on the one hand, and, on the other, the small number of individuals suffering [adverse reactions and events] poses an ethical and practical dilemma… Leaving those individuals and their families to bear the costs of their injuries would mean that the community would benefit from these individuals’ contributions to herd immunity, leaving the uninjured to receive a health benefit at the cost of the injured”. Compensation schemes are important for gaining public confidence and compliance regarding vaccine uptake, and ensuring government accountability (Attwell et al., 2024; Attwell, Hannah, et al., 2022; Centre for socio-legal studies, 2024; Halabi et al., 2023). It has been argued that these schemes are part of good vaccine policy and surveillance safety systems, which are viewed positively by communities, because they counter concerns about adverse events (Liu Shiu Cheong et al., 2024).
The Australian COVID-19 Vaccine Claims Scheme
The Australian COVID-19 Vaccine Claims Scheme provides financial compensation for individuals who experience one of a defined number of serious adverse events (see Table 1). At inception, the Scheme did not quantify what constituted an eligible scope of reactions (Madden and Cockburn, 2021) but, over time, the scope has been narrowed to the events detailed in Table 1. As we will demonstrate in this paper, the restrictive nature of the scheme and its further tightening over time has occurred in parallel with a growth in the evidence on the breadth of adverse reactions that can occur due to all COVID-19 vaccines available in Australia.
In international consumer law, individuals injured by a product are entitled to compensation from the manufacturer for harms (present and future). However, as part of the global rollout of COVID-19 vaccinations at the height of the pandemic, the Australian Government agreed to the indemnification of vaccine manufacturers. This mean that citizens who experience adverse events have no legal recourse against vaccine manufacturers. The vaccine compensation scheme was designed as an answer to this accountability problem.
The total number of adverse COVID-19 vaccine events in Australia remains unclear, however the open Database of Adverse Events in Australia (OpenDEAN Database, 2024) (extracted from the TGA website) lists over 139,000 reports of adverse events following Pfizer, Moderna and AstraZeneca, 22,000 serious cases and close to 1,000 deaths. Underreporting is standardly acknowledged as a general issue with pharmacovigilance systems (Lazarus 2010). In the case of the COVID-19 vaccines’ pharmacovigilance reporting, it is also documented by users of the system that medically confirmed serious adverse events have either not been written up in TGA documentation, or not been followed up by TGA to record further diagnoses and on-going or long-term impacts following initial report submission (COVERSE 2022).
International data on serious adverse events to COVID-19 vaccines ranges from ‘very rare’ to as much as 1 in 800 doses (Buergin et al., 2023; Fraiman et al., 2022; Graham, 2023). In terms of the compensation scheme, it was revealed in Senate Estimates (Senate Community Affairs Committee, 2024) in early 2024 that 4,282 claims have been submitted to the Australian COVID-19 Vaccine Claims Scheme, with 3,533 claims finalised, of which only 324 were paid out, while the majority have been rejected or withdrawn (on advice from Services Australia). 749 claims were outstanding at the time of the hearing. This equates to less than 10% of applications being successful, even though as of 2022 individuals can only apply if they have one the reactions approved for compensation. The Scheme was set to be closed in early 2024 but was subsequently extended to September 2024, while the COVID-19 vaccine program itself is set to continue for the foreseeable future. The Scheme closure leaves no recourse whatsoever for COVID-19 vaccine-injured citizens to access compensation for life-changing injuries.
| Vaccine | Reaction compensated |
|---|---|
| AstraZeneca | Capillary Leak Syndrome |
| Cerebral Venous Sinus Thrombosis | |
| Guillain Barre Syndrome | |
| Thrombocytopenia / Immune Thrombocytopenia | |
| Transverse Myelitis | |
| Anaphylaxis | |
| Pfizer, Moderna & Novavax | Erythema Multiforme (Major) |
| Myocarditis | |
| Pericarditis | |
| Anaphylaxis |
Findings
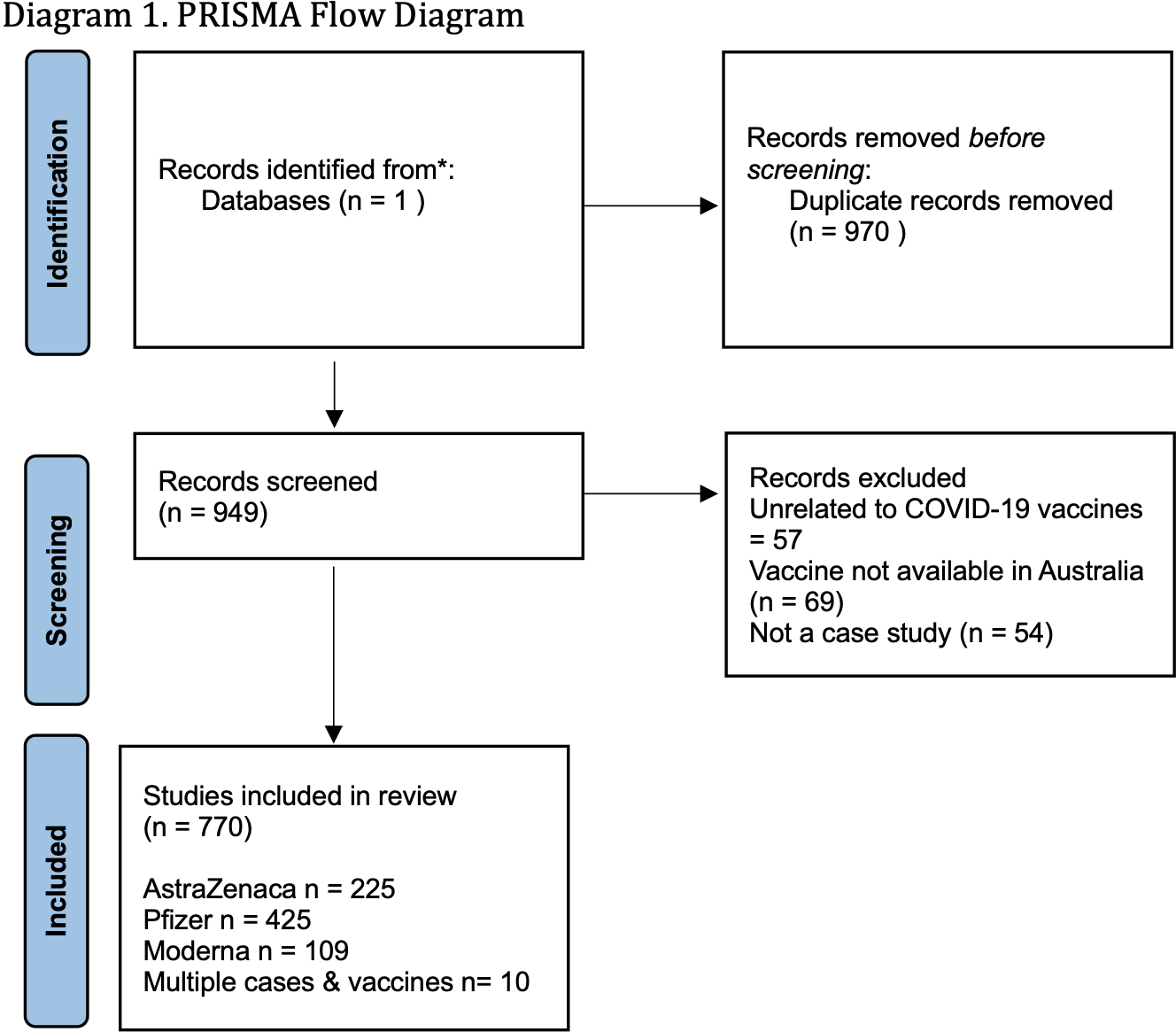
In Table 2, below, we present the findings of the systematic review, grouped by medical theme. The review found a much greater array of reactions in the literature than approved for compensation. In total 970 papers were identified and 771 included in the final review. The majority of cases examined a single individual, but some papers examined up to 25 patients. The final set of 771 papers were read in full, and organised into broad medical categories as well as the specific reactions outlined in the compensation scheme (unless zero cases were found).
All reactions were ‘serious’, i.e. requiring medical intervention and hospitalisation, and met the bar of being worth documenting in the medical literature.
While the sample of case studies is not presumed to be representative, it is nonetheless interesting that such a similar array of events were found across the different vaccine types (despite the limiting of reactions particularly under Pfizer and Moderna to just four: anaphylaxis, inflammatory disorders of the hearts – myocarditis and pericarditis, and the skin condition erythema multiforme). Also, a higher proportion of adverse events were found for some reactions not listed on the compensation scheme (e.g. kidney and eye disease) than the conditions which are listed.
| Vaccine brand | Number of case reports/series | Reaction in the case study literature | Studies | Included in the Scheme? |
|---|---|---|---|---|
| AstraZeneca | 234 | Cerebral Venous Sinus Thrombosis | 10 | Yes |
| Guillain Barre Syndrome (GBS) | 17 | Yes | ||
| Neurological disorders (severe) excluding GBS | 16 | No | ||
| Haemorrhagic disease | 5 | No | ||
| Ischemic Stroke | 5 | No | ||
| Inflammatory disorders of the brain, nervous system, organs and full body systems | 36 | No | ||
| Myocarditis | 5 | No | ||
| Heart irregularities and events (other) | 4 | No | ||
| Skin reactions | 22 | No | ||
| Thrombocytopenia | 33 | Yes | ||
| Thrombotic illnesses (other) | 24 | No | ||
| Transverse myelitis | 8 | Yes | ||
| Vasculitis | 9 | No | ||
| Vision damage, blindness and eye disease | 22 | No | ||
| Other | 18 | No | ||
| Pfizer | 441 | Cerebral Venous Sinus Thrombosis | 4 | No |
| Guillain Barre Syndrome (GBS) | 10 | No | ||
| Neurological disorders (severe) excluding GBS | 40 | No | ||
| Diabetes | 4 | No | ||
| Kidney disease (including damage, chronic and organ failure) | 38 | No | ||
| Meningitis | 6 | No | ||
| Myocarditis | 31 | Yes | ||
| Pericarditis | 5 | Yes | ||
| Heart irregularities and events (other) | 13 | No | ||
| Serious inflammatory disorders of the brain, nervous system, organs or full body systems | 34 | No | ||
| Skin reactions | 57 | No | ||
| Erythema Mutliforme | 3 | Yes | ||
| Thrombocytopenia / immune thrombocytopenia | 20 | No | ||
| Thrombotic illnesses (other) | 5 | No | ||
| Thyroid disorder | 14 | No | ||
| Vasculitis | 14 | No | ||
| Vision damage, blindness and eye disease | 47 | No | ||
| Other | 96t | No | ||
| Moderna | 110 | Guillain Barre Syndrome (GBS) | 5 | No |
| Neurological disorders (severe) excluding GBS | 10 | No | ||
| Kidney disease (including damage, chronic and organ failure) | 10 | No | ||
| Myocarditis | 11 | Yes | ||
| Heart irregularities and events (other) | 4 | No | ||
| Serious inflammatory disorders of the brain, nervous system, organs or full body systems | 5 | No | ||
| Skin reactions | 12 | No | ||
| Thrombocytopenia / immune thrombocytopenia | 8 | No | ||
| Thrombotic illnesses (other) | 6 | No | ||
| Thyroid disorders | 5 | No | ||
| Vasculitis | 4 | No | ||
| Vision damage, blindness and eye disease | 6 | No | ||
| Other | 25 | No |
Where cases reported multiple vaccines and injuries, or multiple injuries from the same vaccine, they are included in both/all categories. Case study numbers therefore do not represent total patients. Categories included represent those listed in the compensation scheme (except where zero case studies were found), and categories with more than 4 reports. See Appendix 1 for methodology.
Our review demonstrates a gross misalignment between the adverse reactions covered by the Scheme, and those found in the peer-reviewed medical literature. Our Pubmed search revealed a much broader range of serious reactions than covered by the compensation scheme, indicating that the Scheme design has not kept pace with knowledge about serious adverse events.
Consistent messaging around the COVID-19 vaccines have been that they are safe and effective, including from the Australian Therapeutic Goods Administration (Australian Theraputic Goods Administration, 2024; Hotez, 2023; WHO, 2020). Yet the number of vaccine injury applications combined with the adverse event literature presented in this systematic review shows that serious adverse reactions do happen, resulting in a wide range of health issues. The restriction of the Scheme through its tight construction could be viewed as a means to quieten counter-narratives that might disrupt government rhetoric regarding the safety of the vaccines – politically, one can argue that few applicants presents an optic of few adverse events. Minister Bill Shorten has used the low number of approved compensation applications precisely in this way to argue for vaccine safety (Galloway, 2023). Here, the exclusionary nature of the Scheme potentially plays an agenda management role. Agenda management is a process whereby political actors attempt to block issues transitioning from the public sphere to the institutional agenda of the government sphere, or, failing that, attempt to control the government sphere agenda (Dercy 2000; McConnell 2003). To enact agenda management, political actors actively shape the definition and framing of issues to make them as innocuous as possible and delegitimise individuals and organisations agitating for change (Bromfield et al 2024; Bali and Halpin 2021). Restricting the scheme manages the public and government agenda, using discursive strategies that define and frame both the vaccine policy problem and any solutions to it as minor problems.
In addition to the identified operative misalignment, the Scheme is administratively burdensome and problematic in a range of ways. The application form for the COVID-19 Vaccine Claims Scheme excludes individuals at the outset by asking applicants to tick one of the defined boxes for reactions and provide letters from specific specialists for each reaction type. Applicants have no room to present medically confirmed serious adverse events that are different to the pre-defined shortlist on display, such as those found in the systematic review presented in this paper. Brown et al (2021) has demonstrated the ways that application forms are constructed in ways that are deliberately exclusionary (see also Meers, 2020).
Scheme applicants submit up to 1,000 pages of paperwork and are waiting between 200 and 450 days for responses from government (House of Representatives, 2024). As of 2023, only $7.2m of the $76.9m projected for payments had been paid (Ward, 2023). Media reports indicate that those seeking compensation find the process stressful and are often seriously ill (Ward, 2023), making these long and burdensome application processes difficult or impossible to navigate. Other reports say the application process is humiliating and degrading (Ward, 2022), indicating significant psychological burdens associated with the scheme.
The indemnities provided by the Commonwealth Government in the advance purchase orders that it negotiated with vaccine manufacturers represent a financial liability to the Commonwealth Government (Commonwealth Department of Health, 2021; Commonwealth Government, Budget Papers, 2021). The rationale in favour of the indemnities was related to the ‘timely supply’ (Commonwealth Department of Health, 2021) of vaccines during the pandemic. In this context, the exclusionary nature of the scheme, combined with the administrative burden of accessing compensation, represents a significant risk-transfer from the Commonwealth Government onto those who have been injured by the vaccine. The result is that neither the manufacturer nor the procuring government actually bear the risk burden associated with the rapid development and rollout of COVID-19 vaccines.
The exclusionary nature of the scheme has received on-going media attention (Graham, 2023; Ward, 2022, 2023), and likely has negative implications for trust in government and the on-going COVID-19 vaccination program, as well as vaccine uptake rates more broadly. The Australian Government is not providing a meaningful vaccine compensation scheme, and is currently not properly accountable for adverse events and harms caused by COVID-19 vaccines. The tightly restricted scheme allows government to ‘perform’ accountability and a social safety net around vaccine adverse events, without meaningfully delivering on this promise.
We call for an immediate review of the Compensation Scheme to align it first of all with the medical evidence base of wide-ranging serious reactions. Secondly, a revised scheme should align meaningfully with the scalar losses of health and income experienced by Australians whose doctors and specialists have (drawing on this literature) recognised their adverse reactions as owing to their COVID-19 vaccination, but who are ineligible for the current Australian Scheme. Australians with medically confirmed serious adverse reactions should have the opportunity to apply to a revised compensation scheme, with no further penalties to themselves.
References
Amer SA, Al-Zahrani A, Imam EA, et al. (2024) Exploring the reported adverse effects of COVID-19 vaccines among vaccinated Arab populations: a multi-national survey study. Scientific Reports 14(1): 4785.
Attwell K, Hannah A and Leask J (2022) COVID-19: talk of ‘vaccine hesitancy’ lets governments off the hook. Nature 602(7898): 574–577.
Attwell K, Turvey J and Wood L (2024) COVID-19 vaccination of at-risk and marginalised groups: recentering the state in vaccine uptake. Social Science & Medicine 348: 116812.
Australian Theraputic Goods Administration (2024) COVID-19 Vaccines. Available at: https://www.health.gov.au/our-work/covid-19-vaccines/is-it-true
Brown JT, Carey G and Malbon E (2021) What is in a form? Examining the complexity of application forms and administrative burden. Australian Journal of Public Administration 80(4): 933–964.
Buergin N, Lopez‐Ayala P, Hirsiger JR, et al. (2023) Sex‐specific differences in myocardial injury incidence after COVID‐19 mRNA ‐1273 booster vaccination. European Journal of Heart Failure 25(10): 1871–1881.
Centre for socio-legal studies (2024) Covid-19 NFCS – Global Summary. University of Oxford. Available at: https://www.law.ox.ac.uk/nofault-compensation-schemes-for-covid-19-vaccines/covid-vaccine-nfcs-downloads
Commonwealth Departmet of Health (2021) Commonwealth Department of Health Submisson to the Vaccine Indemnity BIll. Canberra: Commonwealth Department of Health.
Commonwealth Goverment, Budget Papers (2021) Budget Release 2020-2021. Canberra, Australia: Commonwealth Government of Australia.
COVERSE (2022) Vaccines, Long Vaccine Syndrome, and Long Covid: A submission by COVERSE Ltd to the Australian Parliament Inquiry into Long Covid and Repeated Covid Infections. Canberra: COVERSE. Available at: https://media.coverse.org.au/documents/long-covid-inquiry/516 – COVERSE.pdf
Department of Health and Aged Care (2023) COVID-19 Vaccine Claims Scheme – Policy. Canberra: Commonwealth Government.
Fraiman J, Erviti J, Jones M, et al. (2022) Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 40(40): 5798–5805.
Galloway A (2023) Less than 50 payments made for adverse reactions to COVID-19 vaccines. Sydney Morning Herald. Available at: https://www.smh.com.au/politics/federal/less-than-50-payments-made-for-adverse-reactions-to-covid-19-vaccines-20220915-p5bigu.html
Graham C (2023) A Numbers Game: From Heaven To Hell In The Shadow Of A Vaccine. New Matilda. Available at: https://newmatilda.com/2023/08/26/fear-and-loathing-and-the-numbers-games-from-heaven-to-hell-in-the-shadow-of-a-vaccine/
Halabi S, Ginsbach K, Gottschalk K, et al. (2023) No-Fault Vaccine Injury Compensation Systems Adopted Pursuant to the COVID-19 Public Health Emergency Response. EMORY INTERNATIONAL LAW REVIEW 37(55): 60–99.
Hassan YAM, Daud Ali M, Al-Eid RR, et al. (2022) A retrospective evaluation of side‐effects associated with the booster dose of Pfizer-BioNTech/BNT162b2 COVID‐19 vaccine among females in Eastern Province, Saudi Arabia. Vaccine 40(49): 7087–7096.
Herd P and Moynihan D (2019) Administrative Burden: Policymaking By Other Means. New York: Russel Sage Foundation.
Herd P and Moynihan D (2021) Health care administrative burdens: Centering patient experiences. Health Services Research 56(5): 751–754.
Hotez P (2023) The Deadly Rise of Anti-Science: A Scientist’s Warning. Baltimore: John’s Hopkins University Press.
House of Representatives (2024) Hansard. Commonwealth Government of Australia.
HRSA (2024) About the National Vaccine Injury Compensation Program. Available at: https://www.hrsa.gov/vaccine-compensation/about
Hunt G (2021) No Fault COVID-19 Indemnity Scheme. Available at: https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/no-fault-covid-19-indemnity-scheme
Keiser LR and Miller SM (2020) Does Administrative Burden Influence Public Support for Government Programs? Evidence from a Survey Experiment. Public Administration Review 80(1): 137–150.
Kutlesa NJ (2004) Creating a Sustainable Immunization System in Canada – The Case for a Vaccine-Related Injury Compensation Scheme. Health Law Journal; Edmonton 12: 201–42.
Lazarus R (2010) Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS). Washington, USA.
Liu Shiu Cheong D, Tran J, Chong W, et al. (2024) Attitudes, perceptions, and experiences of Western Australians towards vaccine safety surveillance systems following COVID-19 vaccines: A qualitative descriptive study. Australian and New Zealand Journal of Public Health 48(1): 100108.
Madden B and Cockburn T (2021) What’s the new COVID vaccine indemnity scheme? Two legal experts explain. The Conversation. Available at: https://theconversation.com/whats-the-new-covid-vaccine-indemnity-scheme-two-legal-experts-explain-163717
Nasreen S, Calzavara A, Buchan SA, et al. (2022) Background incidence rates of adverse events of special interest related to COVID-19 vaccines in Ontario, Canada, 2015 to 2020, to inform COVID-19 vaccine safety surveillance. Vaccine 40(24): 3305–3312.
OpenDEAN Database (2024). Available at: https://opendaen.info/
Ray V, Herd P and Moynihan D (2023) Racialized Burdens: Applying Racialized Organization Theory to the Administrative State. Journal of Public Administration Research and Theory 33(1): 139–152.
Senate Community Affairs Committee (2024) Canberra: Official Recording of Senate Committee Proceedings from the Australian Parliament. Available at: https://www.aph.gov.au/News_and_Events/Watch_Read_Listen/ParlView/video/2509931?startTime=18610
Wang J, Jing R, Lai X, et al. (2020) Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 8(3): 482.
Ward M (2022) ‘Humiliating and degrading’: Patients wait months for answers on vaccine injury claims. Sydney Morning Herald. Sydney. Available at: https://www.smh.com.au/national/humiliating-and-degrading-patients-wait-months-for-answers-on-vaccine-injury-claims-20221117-p5bz9m.html
Ward M (2023) Thousands left waiting for compensation after claims of COVID-19 vaccine injury. Sydney Morning Herald. Sydney. Available at: https://www.smh.com.au/national/thousands-left-waiting-for-compensation-after-claims-of-covid-19-vaccine-injury-20230413-p5d03y.html
WHO (2020) COVID-19 vaccines: safety surveillance manual. World Health Organisation.
Wood N, Macartney K, Leask J, et al. (2020) Australia needs a vaccine injury compensation scheme: Upcoming COVID-19 vaccines make its introduction urgent. Australian Journal of General Practice. ajgp.covid36
Appendix One: Methods
The research sought to investigate the alignment of the COVID-19 vaccination scheme with the state of evidence on adverse events. To do this, we did not seek a fully comprehensive sample of adverse events but rather investigated the breadth of clear, data-supported reactions by vaccine type, to determine if the categories approved for compensation accurately reflected patients’ experiences in the peer-reviewed literature. Given the size of the adverse events literature, a single database was used (Pubmed), and results were limited to peer-review case study reports of adverse events affecting one or more individuals.
The following search string was run in PubMed, and includes the main vaccines available in Australia and listed on the compensation scheme. Novavax was excluded because it is only compensated for anaphylaxis according to the current Scheme application form.
Search string: ((“mRNA-1273″[Title/Abstract] OR “Moderna”[Title/Abstract] OR “SpikeVax”[Title/Abstract] OR “Elasomeran”[Title/Abstract] OR “BNT162b2″[Title/Abstract] OR “Pfizer”[Title/Abstract] OR “Comirnaty”[Title/Abstract] OR “ChAdOx1″[Title/Abstract] OR “AstraZeneca” [Title/Abstract] OR “Vaxzervia*” [Title/Abstract]))
Inclusion Criteria
Dates: 2021-2024
Humans
Peer-reviewed
English
Case study/report of adverse vaccine event (one or more individuals)
Exclusion criteria:
Not published in English
Not a peer-reviewed publication
Not a case report
Not a vaccine available in Australia
Systematic reviews of case studies
Results of the systematic review are presented in Diagram 1 (PRISMA Flow Diagram). In total 970 papers were identified and 771 included in the final review. The majority of cases examined a single individual, but some papers examined up to 25 patients. The final set of 771 papers were read in full, and organised into broad medical categories as well as the specific reactions outlined in the compensation scheme (unless zero cases were found).
Media about this report
- Sydney Morning Herald, 25th August 2024, Payments for COVID vaccine injuries are ending. Patients want that changed
- Sydney Morning Herald editorial, 25th August 2024, Willingness to review and learn necessary for post-COVID response
- news.com.au, 28th August 2024, ‘1000 pages’: Aussie dad’s ‘cruel’ three-year wait for vaccine injury compensation
- The Mandarin, 12th September 2024, Keep the COVID-19 vaccination claims scheme open